Survey of the Solar System
422 - 428
As you will soon see, the SNT naturally explains many of the observed properties of the solar system. However, the "Devil is in the Details" and there remain many unanswered riddles concerning the formation of the planets and other bodies in the solar system. In this section we will list some of the most important characteristics of the Solar System. In subsequent sections we will look more closely at how the SNT explains (or fails to) these facts.
Looking at the Facts - Orbital Characteristics
Table 16.1 summarizes some of the most basic properties of the planets and their orbits:
|
|
|
|
| Table 16.1 Summary of orbital characteristics of the planets |
Example 16.3 Argue that the data summarized in Table 16.1 is evidence supporting the SNT.
Solution:The SNT predicts that since the planets from from a rotating accretion disk they should have the same direction of motion both as they revolve around the sun and as they rotate on their individual axes of rotation. This is borne out by the evidence.
Planetary Types
The solar system contains two very distinctly different kinds of planets.
|
 |
||||||
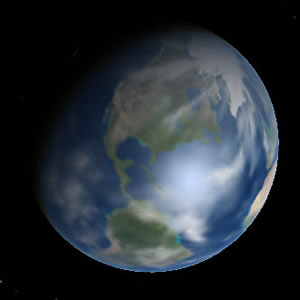
Figure 16.2 Earth - one of the Terrestrial Planets |
|||||||
|
 |
||||||
Figure 16.3 Jupiter - one of the Jovian planets.
|
Other Bodies in the Solar System
Between the orbits of Mars and Jupiter lies a region of "space junk" - leftovers from the earliest stages of planetary formation. This is the asteroid belt and asteroids or minor planets are rocky bodies that range in size from large boulders to bodies measuring hundreds of kilometers across. More than 100 000 asteroids are known and several thousand of these travel on Earth-crossing orbits. This means that their orbits around the sun bring them close enough to Earth that a collision is possible. This is an extremely important feature of how objects in the solar system interact and will become a common motif in our discussion of processes in the solar system.
Figure 16.4 shows the location of the asteroids. If you look carefully at this image you will also note two groups of asteroids called the "Trojans" and "Greeks". These are asteroids whose orbits are "locked" in a special relationship between the combined gravitational effects of Jupiter and the Sun.
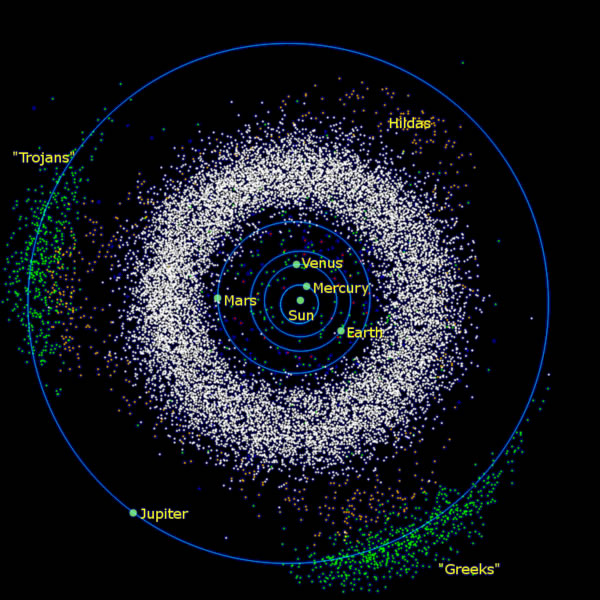 |
| Figure 16.4 The asteroid belt also showing the location of the Trojan and Greek asteroids. |
Further out from the asteroid belt and outside the orbit of Neptune lies the Kuiper Belt - a region populated by small, dark icy bodies. There are millions of these bodies ranging in size from meters across to more than a few hundred kilometers. We now classify Pluto as a dwarf-planet and believe that it is an example of a verify large Kuiper Belt Object (or KBO). Figure 16.5 shows the Kuiper Belt. While the asteroid belt is likely material from a "failed planet" the Kuiper Belt material consists more of water and carbon dioxide ices and represents some of the very earliest material in the solar system. Sophisticated computer models of the formation of planetary systems predict that such materials should form in the outer most reaches of a planetary system. |
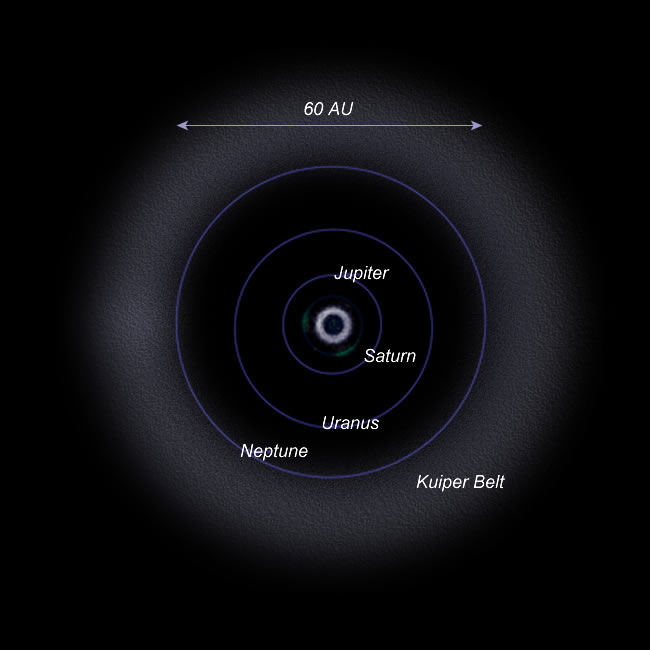 |
| Figure 16.5 The Kuiper Belt |
Comets and Meteors
One of the most dramatic sights in the night sky is provided by a bright comet. Every several years Canadian skies are graced by the appearance of one of these "ghostly" interlopers. Figure 16.6 shows two recent comets - Hyakutake (1996) and Holmes (2007).
 |
 |
| Figure 16.6a Comet Hyakutake as it appeared high overhead in the winter of 1997 | Figure 16.6b Comet Holmes, November 2007 (images courtesy The King's University College Observatory) |
As you will learn in Chapter 19, some of the comets originate in the Kuiper belt while others come from the Oort Cloud - a vast and much more distant reservoir of icy material left over from the formation of the solar system.
The night sky is also witness to another surprise visitor - the meteor. The vast majority of the meteors that you see are nothing more than tiny specks of either rocky material or icy debris left behind by comets. As the parent body or meteoroid enters the Earth's atmosphere the friction between the atmosphere and the meteoroid generates an enormous amount of heat and vapourizes the meteoroid to produce the flash of light that we see as a meteor. As you will also learn in Chapter 19, there is an intimate connection between asteroids, comets and meteoroids.
Age
Have you ever heard the expression "old as the hills"? Just how old are the hills? How old is the earth? The presently favoured age for the earth is 4.5 billion years. How do we know this?The most useful method of determining these ages uses the technique called radioactive dating . Some elements are unstable and spontaneously split into lighter elements. This gives off energy and leaves behind "daughter" elements that, if they are stable, will slowly accumulate. THe basic idea behind radioactive dating methods is to compare ratios of abundances of mother/daughter elements. From this the amount of time needed to produce the observed abundance ratio can be determined.
Suppose you took a pure lump of Uranium and used it as a paper holder. The French physicist Henri Bequerel did this in 1895 to hold photographic (light sensitive) paper in a drawer. When he used the paper and developed the picture he also had a distinct outline of the rock! He had discovered radioactivity . Had he waited about 700 million years he would have found that his lump of uranium-235 was now half lead . Radioactive elements slowly turn into lighter elements and the time required for half of the "mother" element to turn into the "daughter" element is called the half-life of the element. Half-lives can be measured very precisely in the laboratory by measuring the amount of radiation given off by a radioactive sample each second.
Suppose you find a rock that has 7 times as much lead as uranium - how old is the rock? (assume there was no lead originally present in the rock and that it all came from the radioactive decay of the Uranium)
time(half lives.) |
Fraction
that is Uranium-235 |
Fraction that is Lead-207 |
1 |
1/2 |
1/2 |
2 |
1/4 |
3/4 |
3 |
1/8 |
7/8 (7 times as much lead) |
This would take 3 half lives or about 3 x 700 Ma = 2.1 billion years
In the applet Half Life (Figure 16.7) the "mother" element is shown in red, the "daughter" element is shown in green. Blue represents stable element(s). In practice, 2 or more different minerals are used to compare the ratios of daughter/mother species and from this determine the age of the rock sample. The term "age" needs some explaining. This refers to the time at which the chemical composition that you are measuring was set. The time of solidification could mark the beginning of our time measurement, for example. |
|
| Figure 16.7 |
Example 16.4 Nickel-56 (Ni-56) is a radioactive isotope of Nickel that is produced in Type I supernovae. The half-life of Ni-56 is 6.1 days. If 1 solar mass of Ni-56 is produced in a supernovae explosion estimate how much Ni-56 remains 1 month after the initial explosion.
Solution: One month is close to 5 half-lifes for Ni-56. The amount of Ni-56 remaining is (1/2)(1/2)(1/2)(1/2)(1/2) = 1/32 or about 3%. After one month only about 0.03Mo of Ni-56 remains. Most of the Ni-56 has been turned into another radioactive element Cobalt-56 which decays with a half-life of 77 days.
So ... How old is the solar system?
By using a number of different dating techniques (Potassium-Argon, Uranium-238 and Rubidium-Strontium) astronomers have found the following table:
| oldest rocks on Earth known are zircon crystals from Australia | age is 4.3 billion years (4.3 Ga) |
| lunar rocks from Apollo lunar missions | age 4.48 Ga |
| meteorites | age 4.56 Ga |
| Table 16.2 Ages of different components of the solar system | |
The data from Table 16.2 suggest that the solar system is 4.6 billion years old.
Practice
- List 3 pieces of evidence that argue that the planets and other parts of the solar system formed from a common accretion disk.
- Give at least 3 significant differences between Terrestrial and Jovian planets.
- A rock formed 2.8 billion years ago. What fraction of its original Uranium-235 would remain today?
- When Edwin Hubble discovered the law of universal expansion the value of the Hubble constant Ho was about 550 km/s/Mpc. Why can't it be this big? (Hint: recall from Chapter 15.1 that the age of the universe is given (approximately) by the expression t = 1/Ho)

To understand the evidence that supports the SNT
Chp 19.1

